Abstract
Introduction
Differentiated thyroid cancer (DTC) is the most common endocrine malignancy. Treatment options include surgery, radioiodine & hormonal therapy. Radioiodine therapy (RAI) is often used after surgery with the purpose of ablation of residual thyroid tissue, adjuvant therapy or clinically evident metastatic disease. However, the use of RAI carries a risk of secondary malignancies, such as leukemias.
The 10-yr survival rate of patients with DTC > 90%. However, several studies showed those patients may be at risk for secondary cancers.
Therapy related myeloid neoplasm (tMN) can develop after RAI exposure.
The relationship between thyroid and myeloid neoplasm (MN) may not be fully explained by exposure to RAI.
In this retrospective study, we investigated the association between MN & DTC.
Methods
This study included patients >18years with DTC and either MN ((Acute Myeloid Leukemia (AML), Myelodysplastic syndrome (MDS), Chronic myelomonocytic leukemia (CMML), or Myeloproliferative Neoplasms (MPN) who had been diagnosed and/or treated at UTMD Anderson Cancer Center from 1/2012-12/2020
Descriptive statistics were performed to define the demographic, clinical and biological characteristics of the patients included in the study.
Results
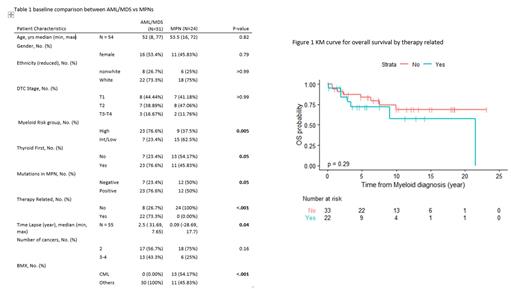
Fifty-four pts had both DTC and MN. The median age was 52 yrs (18-77). Most of the patients were white 41/54 (75.9%) with equal sex distribution and of the papillary subtype of DTC 48 (88.8%). DTC stages were I (15(42.86%), II -15 (42.86%) III & IV- 5 cases (14.29%). Of the MN patients, AML/MDS cases were 30 (56.36%), while MPNs cases were 24(43.64%). Majority were CML 13/24 (54%) (p =0.01). The most common cytogenetics karyotypes detected were diploid in 17 (30.91%) followed by complex in 8 patients (14.55%). The most frequent positive mutation was FLT3 in 6 cases (10%) followed by TP53 and ASXL1 with 5 (9.26%) patients respectively. While the mutation panel were negative in 16 patients (29.63%).
RAI was used in 42(76.36%) patients, with a median dose 142 mCi (75-290). Thirty patients (70%) of those treated with RAI developed therapy related MN, while 12 patients had MN earlier occurred first in 34 patients (61.82%) (proceeded AML/MDS in 23 (74.19%) and 11(45.83%) (P= 0.005) in cases of MPNs. The median time lapse from developing thyroid to myeloid cancer was 4 years. Thyroid cancer proceeded AML or MDS by 2.5 years, while thyroid cancer happened around the same year (0.09) with MPNs (p= 0.04) Table 1
A hypomethylating agent (HMA) based therapy for myeloid neoplasm was the most commonly used in 15 (42.86%), other therapies included cytarabine (5), cladribine (4) & fludarabine (3) based .37 pts (67.27 %) achieved complete remission, while 18 (32.73%) underwent stem cell transplantation.
Overall survival showed that 40 (74%) were still alive. Therapy related AML/MDS Overall survival were inferior to non-therapy related patient (p= 0.29) figure 1. Patients who had 3 or 4 types of cancer to the group who had only thyroid and myeloid cancer showed increase risk of death with a hazard ratio of 1.72 (0.62-4.77 however this was not statistically significant also (p= 0.30).
Conclusion
In our cohort patients who were exposed to Radioiodine (RAI) had developed therapy related myeloid neoplasm (t-MN) AML/MDS even with doses < 100 mCi. Median to develop neoplasm (t-MN) were 4 years, complex cytogenetics is frequent. No statistically significant difference in survival between therapy related and none therapy related group. DTC did occur with MPNs as well with CML most frequent subtype. Prospective studies need to be done to further elucidate the relationship between DTC & MPN.
Pemmaraju: ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Affymetrix: Consultancy, Research Funding; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Cellectis S.A. ADR: Other, Research Funding; CareDx, Inc.: Consultancy; LFB Biotechnologies: Consultancy; MustangBio: Consultancy, Other; Plexxicon: Other, Research Funding; Aptitude Health: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Incyte: Consultancy; Sager Strong Foundation: Other; Daiichi Sankyo, Inc.: Other, Research Funding; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Consultancy; Samus: Other, Research Funding; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; Springer Science + Business Media: Other; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy; Roche Diagnostics: Consultancy; Clearview Healthcare Partners: Consultancy; Blueprint Medicines: Consultancy; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy. Sasaki: Novartis: Consultancy, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees. Verstovsek: Promedior: Research Funding; PharmaEssentia: Research Funding; NS Pharma: Research Funding; Ital Pharma: Research Funding; Incyte Corporation: Consultancy, Research Funding; Gilead: Research Funding; Genentech: Research Funding; CTI BioPharma: Research Funding; Celgene: Consultancy, Research Funding; Blueprint Medicines Corp: Research Funding; AstraZeneca: Research Funding; Protagonist Therapeutics: Research Funding; Roche: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; Constellation: Consultancy; Pragmatist: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal